(Remember, when we make a statement and enclose it in parenthesis, nothing will be lost if you ignore it.)
I Words: Most people hardly think that there is a difference between "weight" and "mass" and it wasn't until we started our exploration of space that is was possible for the average person to experience, even indirectly, what it must mean to be "weightless". Everyone has been confused over the difference between "weight" and "density". Which of us hasn't fallen for the old riddle: What weighs more, a pound of lead or a pound of feathers? Now that Astronauts regularly seem to be demonstrating "weightlessness", ( yet we know they still have all of the matter or mass they had when they were on earth,) more of us are beginning appreciate the weight - mass confusion. Like many confusing concepts, after they are finally understood, they are still difficult to explain to others who don't understand. We hope we can explain the difference between mass, weight and density so clearly that you will have no trouble explaining the difference to your students.
Mass: This concept is so basic that, like length and time, it is really impossible to define. Isaac Newton called mass the quantity of matter. We can talk all around it but we will finally have to admit that our words fail. Some say mass is the amount of matter in something (and hope that no one asks: What is matter?). Others say mass is the measure of an object's inertia (which assumes we understand the elusive property of inertia). To add to the confusion, mass is related to an object's inertia but it also is related to how hard objects are attracted to the earth. Better minds than ours have been confused over the meaning of the concept "mass" and even today, better minds than ours contemplate what mass really means. Our way of giving up on the impossible task of defining mass is to say: mass is the measure of the amount of "stuff" in something. This definition is properly confusing and you can work on the meaning of "stuff"! In the metric system mass is measured in kilograms and grams and these will be the units we will most often use. (In the United States today, almost no one knows what the unit of mass is called--it's not the pound. The pound is a unit of weight--more about weight in the next paragraph. The more mass something has, the harder it is to move or, the more sluggish it is. The correct US unit of mass is called the "slug"--short for sluggishness-- but, as we said, almost no one uses this unit today.)
Weight: If you can finally accept the concept mass even if we have been unable to define it, weight is easy: The weight of a mass is the force that the earth pulls on the mass. We hope you have a feeling for what force means (and we will discuss it later). The entire idea of weight can be understood as the force of gravity on something. Usually we spend most of our time on Earth so our weight is the force that the earth pulls on us. If we get further away from the earth, the force the earth pulls on us is less and we weigh less. If you lived on Mars, the above definition would probably change to: "The weight of a mass is the force that Mars pulls on the mass." The whole idea of weight is related to the force of gravity (and we hate just to use the word "gravity" since it can bring up even more confusion). It would be correct to say, no matter where you might be in the universe that "the weight of a mass is the force of gravity on the mass." In the metric system force is measured in newtons hence weight is also measured in newtons. You will learn later that on the surface of the earth, a mass of 1 kilogram weighs 9.8 newtons. (You will probably never learn anywhere that on the surface of the earth, one slug weighs 32.2 pounds--don't worry about it, very few people know this!) The pound is the US unit of force hence the US unit of weight is also the pound. We will use newtons for the unit of force (and weight) almost always in the discussions that follow.
Density: There are two kinds of density, "weight density" and "mass density". We will only use mass density and when we say: "density", we will mean "mass density". Density is mass per volume. Lead is dense, Styrofoam is not. The metric system was designed so that water will have a density of one gram per cubic centimeter or 1000 kilograms per cubic meter. Lead is about 10 times as dense as water and Styrofoam is about one tenth as dense as water.
II Purpose of the Activity:
The purpose of this activity is to investigate the meaning of mass, weight and density by looking at how each might be measured.
III Materials required for the Activity:
At least one box of #1 (small) paper clips, 20 (or more) long thin rubber bands (#19 will work--they are 1/16" thick and 3+" long), drinking straws, a fine tipped marking pen (Sharpie), scotch tape, 40 (or more) 1oz or 2oz plastic portion cups (Dixie sells them in boxes of 800 for less than $10--see if your school cafeteria has them), lots of pennies (to use as "weights"), light string, 20 (or more) specially drilled wooden rulers or cut sections of wooden molding, about a pound or two of each of the following as available: sand, rice, sawdust, fine crushed Styrofoam (place Styrofoam packing or cups in a plastic bag and pound--yes, this can make a mess), lead shot (can this be used with children? Lead shot can be obtained at a gun and ammo shop), any other finely ground solid material which can be used to illustrate a variety of different densities. (We have avoided liquids since they seem to make a bigger mess.)
IV What the teacher must do in advance of the activity:
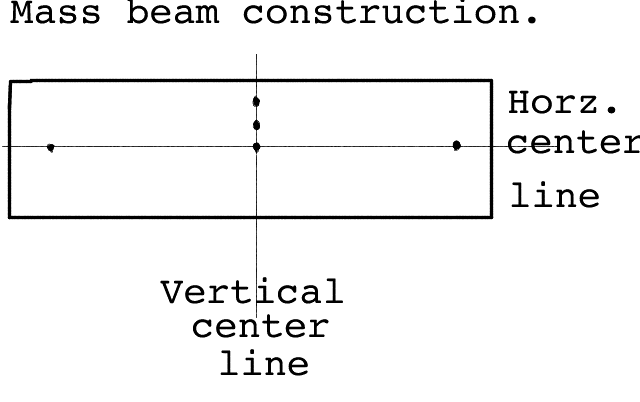
We feel the most difficult preparation item for this activity will be making the drilled ruler or preparing sections of wood properly drilled. (Is it possible to assign tasks like this to parents who have better shop facilities at home?) These will become the balance beams for our mass balance. The actual dimensions of the balance beam is not too critical and a wooden ruler is about right--the best are old ones that have lost the metal strip they often have along one edge. What follows is a description and illustration of how the balance beam should be constructed:
The center hole should be exactly in the center so that when the beam is supported by a nail through this center hole, it will spin around freely. The two end holes should be in line with the center hole and equidistant on each side. Two other holes should be drilled on a line directly above the center hole. The hole size is not critical, about 1/8 of an inch is fine. (We sincerely hope it will not be too difficult for you to accomplish this. We fooled around with metal hangers, etc. but nothing simple seemed to work as well as a carefully prepared stick.)
It will be necessary to poke holes in the portion cups. Should you do this in advance or can your students do it? A small nail works well for this purpose. Probably time could be saved in class if the necessary strings were cut to length in advance (and perhaps even tied to the cups).
Building the "Weight Scale" requires some careful cutting of a straw that can be done with a good pair of scissors or a sharp knife. We think kids can do all of it but it will take time. You should build one prototype Weight Scale in advance so you can work out the details of construction and decide how much of the cutting should be done in advance.
(Once again we remind you that we really don't know the best way to teach these concepts to young students. The following are suggestions for construction and use of the equipment and how we envisioned one might use this stuff but only you can know the best way to present this material to your students.)
Measuring mass: Mass is usually measured with a balance. The idea is to compare the unknown object with the mass of a known amount. Illustrated below is the device we will use to measure mass and we will call it "the Mass Balance".
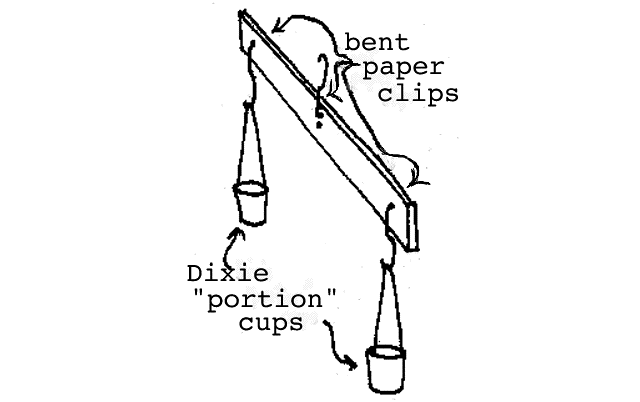 Simple mass balance.
Simple mass balance.
Since everyone seems to have lots of pennies and all pennies are about the same mass, we will use the penny as our standard of mass. (It turns out that the average penny has a mass of about 2.6 grams and you can convert to grams if you wish but for now, we will simply determine mass in "pennies".) The mass measurement is accomplished simply by placing the unknown object in one cup of the Mass Balance and finding out how many pennies placed on the other side it takes to achieve balance. You should first check the Mass Balance with nothing in either cup to see if it is properly "zeroed". You should notice that the balance is most sensitive when the upper paper clip is in the center hole (in fact it is really too sensitive here) and it will be less sensitive when you use the higher holes. Slight errors in the zero reading can be corrected by using shorter or longer string sections on the appropriate side. Make sure all paper clips rotate freely in the drilled holes. The balance will not work properly if the paper clips hang up. The Mass Balance can be loaded with the cups on the table and pulling upward slightly on the support paper clip will test the balance condition. We suggest that students begin by matching pennies on the left with pennies on the right (and they should discover that all pennies aren't really the same--this is real!) After the students become familiar with the use of the balance, we suggest that nearly equal volumes of the assorted materials (sand, rice, metal shot, Styrofoam) be measured. If you are using the 1 oz Dixie portion cups, it is possible to draw a line on the cup 1.4 cm above the bottom and it will represent 10 cubic centimeters and a line 2.3 cm above the bottom of the cup will represent 20 cubic centimeters (or milliliters).
A very important question to consider now is: If you used this Mass Balance on the moon or on Mars, would the same amount of material on one side require the same number of pennies on the other side to balance it as it did on Earth? Naturally there is no easy way for us to perform such an experiment but, having your students think about this should help them to start understanding the difference between weight and mass. Mass or as Newton would say, the quantity of matter in an object, does not change when you change your location in space but, as we will see shortly, weight does change.
Measuring weight: Using a carefully segmented straw, a bent paper clip, a rubber band, some string, a small cup, a 3X5 card and some scotch tape we will construct the "Weight Scale" shown below:
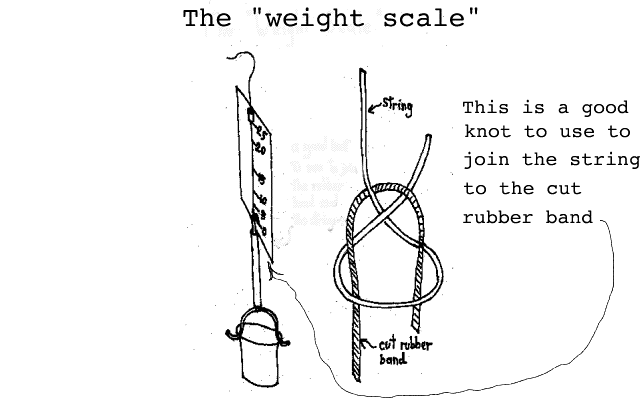
( Since we had difficulty in joining the segment of rubber band to the string, we decided to show you a "carrick bend" which works quite well for this situation. A simple slip knot works well on the bottom where the rubber band attaches to the bent paper clip bail. A later construction detail diagram will give you a better illustration of the Weight Scale.)
The students will calibrate this Weight Scale with pennies and mark the 3 X 5 card with a marking pen during the calibration exercise. Attaching the rubber band to the bail of the cup is easily accomplished with a slip knot but attaching the string to the rubber band is a slight problem--a suggested knot is shown with the illustration. The whole idea is to have the zero of the scale at the bottom of the card using the string-rubber band junction as the pointer. With about 25 pennies in the cup, the rubber band will stretch to about the top of the card. (You hold the scale with the string which has been passed through a small piece of straw taped to the card.) The students will carefully load the cup with pennies and mark the card at about 5 penny intervals.
A more detailed construction of the Weight Scale is shown "below." (Again, we suggest that you construct one in advance so you can evaluate how difficult it is to build.) Note that in the construction diagram "below", we show how the straw should be sectioned so that it can be attached to the 3 X 5 card. (In the final scale, naturally, the string and rubber band fit inside of the straw sections.) It is important that the lower length of the straw be made just long enough to extend from the top of the paper clip "bail" to the bottom of the card with the rubber band sticking out the top. You must be able to tie the rubber band to the string and have the junction of the two be on the lower end of the 3 X 5 with nothing in the cup.
This is the "below" referred to in the above paragraph. We decided it would take a large image to show the necessary details so, if you have the time, click here for Weight Scale Construction Details.
After the students have calibrated the Weight Scale, it might be fun to have them see if they can guess how many pennies have been loaded into their cup by another student. From this they will learn how to read between the marks they have placed on their cards (this is called interpolation) and they will also learn that the scale is really not too accurate. However, all instruments are less than perfect at some level and this crude scale should help them to realize this fact. (We think it is nice that the scale is quite inexpensive and students who wish can construct one at home.)
This Weight Scale can also be used to measure some of the other materials that were measured with the Mass Balance. Hopefully they will find that they will get pretty close to the same answer in "pennies" for the mass as measured on the Mass Balance and the weight as measured on the Weight Scale. (So you ask--what is the difference between weight and mass?) Now comes the key question to ask the class: If you took the Mass Balance and your calibrated Weight Scale to the Moon, do you think they would give the same measurement as on Earth? Remember, you always balance the unknown object against several pennies with the Mass Balance but you just let the unknown object pull down against the calibrated rubber band on the Weight Scale. We hope that this thought experiment will help the students see that the Mass Balance will measure the same no matter where you locate it in space but the Weight Scale, which measures how hard gravity pulls down on the object, will give a smaller reading on the moon. (This is confusing stuff and most college students will have difficulty understanding it. Perhaps if your kids start thinking about it early enough, they may come to a better understanding of the difference between weight and mass when they are older.)
Measuring Density: Since density is mass per volume, the most straight forward way of measuring the density of something is to measure its mass, then measure its volume and divide the mass by the volume. We could do exactly that in this activity but at this point we have no good way to measure volume. If you have a graduated cylinder (they aren't expensive but most elementary schools don't have them) you could use it with some water to mark the small "portion cups" at specific volumes. (We have already suggested that the small 1 oz cups will hold 10 cubic centimeters when filled to a point 1.4 cm above the bottom and it will hold 20 cubic centimeters when filled to a point 2.3 centimeters above the bottom.) Rather than actually measuring the density, we feel it will be sufficient for the students to appreciate that the same volume can be a large mass or a small mass depending upon the material involved. Our plan is to have the same volume of several different materials and measure their mass with the Mass Balance. Hopefully this exercise will help the students to begin to see the relationship between mass, volume and density.
(The next page begins the "Student Activity Sheet". We suggest that these be reproduced in sufficient numbers for the entire class. Whether the students work individually or in groups is best decided by you, however, some of the exercises need at least two people to hold and use the apparatus.)
Which is heavier, a pound of feathers or a pound of lead? If you have never heard this old trick question before--think about it. Now try this one: which takes up more space, a pound of feathers or a pound of lead? Finally, think about this one: which weighs more 100 pennies on the earth or 100 pennies on the moon? The answer to each of these questions requires that you understand the difference between mass, weight and density.
Measuring Mass:
You will measure the mass of objects by comparing them to the mass of pennies with a thing we will call a "Mass Balance". Although mass is usually measured in kilograms or grams, we will measure mass in "pennies". The Mass Balance is shown below.
 This balance measures mass in "penny"
units.
This balance measures mass in "penny"
units.
First test the Mass Balance to see if it is "zeroed". When you lift it by the center paper clip, it should stay fairly level. When the clip is in the top hole, it will balance easily. If you put the clip in the bottom hole, it probably will be too sensitive to balance at all. Test the Mass Balance by placing 5 pennies in both cups and gently lift it off the table--it should balance. Have one student secretly place a number of pennies in one cup and see if you can figure out how many pennies there are in the cup by matching them with pennies in the other cup. (You will find that not all pennies are exactly the same.) After you learn how to use the Mass Balance, you will be given several different materials to measure. Always measure the same volume of the given materials, that is, always fill the material to the same level on the cup on the left and find its mass by placing pennies in the cup on the right. Record your data in a table like the one below:
|
Name of material being measured |
Mass of material in "pennies" |
|
(name first materal meaured here) |
(record its mass in pennies here) |
|
(etc.) |
(etc.) |
|
|
|
|
|
|
Here is an important question to think about: If you took your Mass Balance to the Moon and repeated this experiment, would you get the same result? (Have your teacher discuss with you what mass means--this is quite confusing to many people.)
Measuring Weight:
You will construct and calibrate a Weight Scale. This scale works by measuring how far a certain number of pennies are able to stretch a piece of rubber band. The scale is illustrated below:
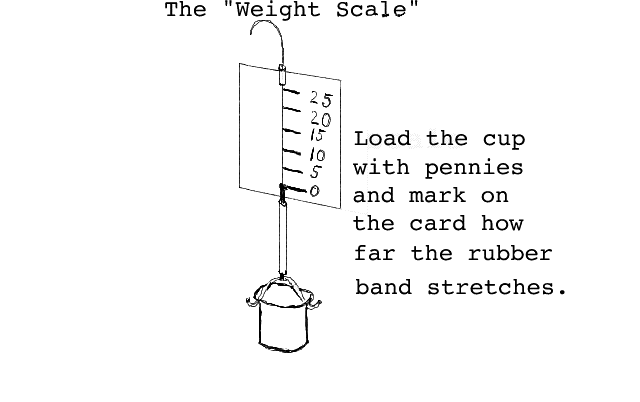 Measuring weight in units of pennies.
Measuring weight in units of pennies.
After you have constructed the Weight Scale (your teacher will give you instructions) you will calibrate it using pennies. While one student carefully holds the Weight Scale by the string the other student will make a line on the card. First mark on the card where the knot touches the card with nothing in the cup (this will be the zero line on the scale). Now add 5 pennies to the cup and carefully mark where the knot touches the card. (Practice with pencil first until you learn how to do this.) Carefully "calibrate" your scale by adding 5 pennies at a time and marking where the knot touches the card. The numbers on your scale will be 0, 5, 10, 15, 20, 25 you probably will not be able to get any more. (Don't expect the numbers to be evenly spaced--rubber bands don't work that way.) If you have calibrated your scale well, you should be able to tell how many pennies someone has placed in the cup without actually counting them. As with the mass balance, you should weigh some of the materials your teacher provides. Always fill the cup with the same volume of material. Make a table like the following:
|
Name of material being weighed |
Weight of material in "pennies" |
|
(give the name of first material weighed here) |
(give the weight of the material in pennies here) |
|
(etc.) |
(etc.) |
|
|
|
|
|
|
Although your measurements are not perfect (no measurements ever are) you can use your calibrated Weight Scale to find the weight of assorted things and you could even use it to count pennies. Now comes a very important question: If you took your calibrated Weight Scale to the Moon, would it work the same way it did on Earth? If you filled it with the same amount of pennies, would the rubber band stretch to the same mark? Have your teacher discuss this with you and see if you can understand the difference between weight and mass.
Measuring Density:
Density tells us how much stuff has been packed into a certain amount of space. Lead is very dense, Styrofoam is not very dense at all. Density can be measured in grams per cubic centimeter. In our experiments, density could be measured in "pennies per cup". In your experiments with the Mass Balance, you always measured the mass of the same volume of material. Use the data you took to list the materials you measured in order of density with the most dense at the top of the list down to the least dense at the bottom of the list. You don't have to calculate the density in "pennies per cup" since you always measured the same volume of material--just look at your data to make the list.
After you have made your list of the densities of the materials, think about the following important question: How do you think the density of a substance would change if you measured it on the Moon rather than on the Earth?
Additional questions to think about:
1. You have an object and you want to know if it will float in water. To answer the question: "will it float?" do you need to know the objects mass, weight or density?
2. A student's mass on Earth is 50 kilograms. If this student went to the Moon, would her mass be more, less, or the same?
3. A student's weight on Earth is 100 pounds. If this student went to the Moon, would he weigh more, less, or the same?
4. A block of wood easily floats on water when on the Earth. If the same block of wood were taken to the Moon, would it float on water? (Really give this some careful thought, most people can not answer this question.)