Replication and translation, the fundamental processes in biology,
involve the separation of double stranded DNA [I]. Duplex-single strand
transition occurs also when DNA is heated[2, 31, upon changing
the buffer sur-rounding the DNA, or under the influence of an external force or
torque[4-61. This so-called denaturing transi-
tion has been extensively studied both in natural and synthetic duplexes. Aside
from its inherent interest, the process is also of primary importance for
applications in the biotech arena. For example, thermal denaturation
is an essential step in the Polymerase Chain Reaction amplification procedure.
For the thermally driven transition of native DNA seg-ments of several thousand base pairs the transition occurs in discrete steps, these steps being determined by the par-ticular base pair sequences [7, 81. In long native DNA the transition is smooth, presumably reflecting a large num-ber of discrete steps occurring at different temperatures. The denaturing transition was also investigated in short oligomers, in the vast majority of cases with random dis-tribution of CG and TA base pairs.
Several
models have been proposed to describe this duplex-single strand transition. The
standard view of
the thermal denaturation of DNA is that it represents
the classic competition between energy and entropy. At low temperatures, the
thermodynamics is dominated by the binding energy of the base pairs. As the
temperature is raised, sections of the DNA separate to take advan- tage of the
greater entropy available to two separated single DNA strands, as opposed to
smaller entropy to
be found in the smaller configuration space accessible to tightly inter-wound
double-stranded DNA. In the ther-modynamic limit (or for circular DNA) melting
transition occurs because of the growth and accretion of denatura-tion bubbles.
For finite size oligomers "fraying" at the ends of a section of
linear DNA is likely to be important as we will discuss below.
Here
we address the simplest possible scenario: the
denaturing of short oligomers, where each strand is a homopolymer (composed of
identical base pairs). Under such circumstances variations of base pair
interaction en-ergies (different for CG and AT pairs) do not occur,
and
the oligomer can be regarded as a
duplex held together
by identical base pair interaction energy at the different sites. We are not
aware of experiments which address the situation where differences between
binding energies as-sociated with different base pairs and other complications do not
arise and which thus would allow the experimental test of simple, but important
descriptions of the melting transition.
For finite oligomers, the following argument can be made: the binding
energy between two bases located at the end of the molecule is smaller than the
binding energy for pairs away from the ends, consequently the unbinding occurs
most likely by a "zipper" like sequential opening of the base pairs,
starting at the ends where the binding is weakest. Such model for DNA melting
has been proposed by C. Kittel [9]. In this so-called zipper model, the melting
of a linear DNA oligomer occurs entirely as a result of strand separation at
the ends. One assigns an energy - ![]() 0 to each
bound base pair, and an entropy equal to S0
to each unbound pair. Then, the partition function of an
N-base-pair oligomer is given by
0 to each
bound base pair, and an entropy equal to S0
to each unbound pair. Then, the partition function of an
N-base-pair oligomer is given by
![]()
where
N1 and N2 are the
number of separated base pairs at the two ends of the linear strand. The zipper
model, and this partition function, ought to be reasonably ac-curate as long as
one can ignore the effects of excluded volume, which should be the case for
oligomers that are not too long, and if the oligomer is uniform. Here, we use
the zipper model as an fitting form for the experimental data. A much simpler
model, assuming that only two configurations occur, completely closed and completely
separated strands, has also been used to describe dena-turing. We call this
model the "two-state" model; for
![]()
this description the appropriate partition function has
the form:
The
two-state model predicts a first order melting tran-sition in the thermodynamic
limit (N![]() ). As it turns
). As it turns
out, the zipper model also leads to
the same conclusion5 [9]. Using the partition functions as given in Eqs. (1)
and (2), physical quantities, such as the average number of paired bases, and
the distribution of oligomers with different numbers of open base pairs, can be
calculated.
The zipper
model is a theoretical scenario in which denaturation takes place via
unraveling at the ends of
a DNA duplex. However, another contributing factor
in the thermal denaturation of long DNA molecules is
the denatured "bubble," a portion of denatured DNA bounded by
duplexed segments. Entropic considerations militate in favor of an accumulation
of such bubbles in a sufficiently long DNA molecule. In fact, the most
phys-ically reasonable picture of the denaturing transition is
in terms of the proliferation and merging of denatured bubbles. Poland and
Scheraga [lo, 111 have proposed a model of the transition based on this notion.
This model admits of elaboration and is amenable to analysis in the context of
field-theoretical approaches to the statistical mechanics of critical
phenomena. It is consistent with either a continuous or a first order
transition, depending on the influence of base pair inhomogeneity [12, 131 and
excluded volume [14]. Furthermore, the Poland-Scheraga model, along with the
closely-related model of Peyrard and Bishop [15],produces results that are
consistent with scaling and hyperscaling analysis of both continuous and first
order transitions [16].
In this paper
we focus on the average number of open base pairs as function of temperature,
using the inten-
sity of the UV absorption at the wavelength of 260 nm. This parameter
predominantly measures base stacking which is directly related to the number of
open base
pairs [17]. Other spectroscopic methods are also avail-able for monitoring the
melting transition. In a separate study [18] we have demonstrated that for the
oligomer dA15/dT15 three different spectroscopic methods (UV absorption, CD
spectroscopy, and a fluorescence based method) give rise to identical (within
experimental error) meltingcurves. We believe therefore that the assumption we
make, namely that the measured UV absorption cor-rectly represents the average
number of open base pairs
is justified.
We have used synthetic poly(A) and
poly(T) oligomers of three different lengths - 15, 30 and 60
bases, PAGE purified, purchased from Operon Technologies. Single strands were
dissolved in 1.5 M Phosphate Buffered Saline (PBS). For recombination, solutions of
comple-mentary strands were mixed in equimolar ratio, warmed up to 90° in a
water bath, followed by a slow cool down to room temperature. This resulted in
complete re-combination of the complementary strands as confirmed with hypochromicity
measurements. A quantity of few pL was isolated from the stock solutions and it
was dis-solved in 500 mL of 50 mM PBS buffer adjusting the
final DNA concentration to 1 OD. For measurement in buffers of higher
molarities the appropriate volumes of
1.5 M PBS were added to the samples
in order to obtain 100 mM and 200 mM buffer concentrations. This led
to a slight dilution of DNA solutions. Absorption mea-surements were done in a
standard quartz cuvette with
a Bekman-Coulter 640 UV/Vis spectrophotometer with an integrated Peltier
heating block and a temperature controller that enable temperature control
between 10°C and 90°C Temperature dependent absorption measure-ments were done
in steps of 1 K. Before the absorption measurement the samples were thermalized
at every tem-perature for 5 min - the time needed for the cuvette and solution
inside to reach the temperature of the heating block. The absorption is smaller
for a DNA duplex than for the same DNA in single strand form [17]; this is re-ferred
to as hyperchromicity. The main component of
this effect is the screening of the intra-base excitations
by dipole-dipole interactions between stacked bases, with significantly smaller
screening for a single strand DNA on which bases are unstacked. For poly
d(A)-poly d(T) this difference, the ratio of the intensity for single strand
and duplex DNA, is 1.4 (see below). In Fig. 1, the tem-perature dependence of
the UV absorption intensity is displayed for three different oligomer lengths.
For all oligomers we observe a smooth transition from the du-plex to the single
strand state, with the transition tem-perature (defined as the half-point of
the transition-see below) and width, increasing with increasing length. The
linear slope visible in the melting curves after the sig-moidal transition
region is a well-known phenomenon at-tributed to residual base uustacking in
the single strands. The linear slope before the transition is indicative of
tem-perature driven conformational changes in the double he-lix; this
phenomenon, known as "premelting," is not well understood [19]. These
phenomena are not accounted for in the models above: the first one is not
related to strand separation, while the degrees of freedom relevant for the
second are not taken into account by the zipper model.
We start with
a comparison between the experimen-
tal results and the two state and zipper models discussed above. Such
comparison is shown in Fig. 1. In the zipper model, the fitting parameter e0‚
(in
Kelvin) was allowed to vary between 6405 and 7090, and the parameter So
was fixed at 20.8. The points utilized for the fit were those closest to the
transition. The dominant tempera- ture dependence of the UV absorption in the
vicinity of the melting transition is due to the separation of base pairs. At
temperatures significantly higher and lower
than the nominal melting temperature, the absorbance exhibits a temperature
dependence as a result of effects that are unrelated to the denaturation of the
DNA, as discussed above.
The fitting results for zipper model
are summarized in
the table below (Table 1). The binding energy increases with salt
concentration, because of ionic screening (the temperature at the midpoint is
).The fact that the two-state model fits the data for the 15mer but not the
60mer

FIG. 1: Temperature dependence of the UV absorption mea-sured at 260 nm for poly d(A)-poly d(T) oligomers of three different lengths -15, 30 and 60 base pairs (bp). All curves are at a molarity of lOOmM.
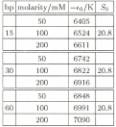
TABLE 1: Parameters used to fit (see text) the measured ab-sorption curves to the zipper model.
indicates that gradual opening of the duplex plays an im-portant role in the melting in the case of larger oligomers.
Figure 2 displays the temperature derivative of the UV absorption. In this case the absorption has been normal-ized so that the integrated weight under each peak is equal to one in all cases. Two curves are shown for each data set. One represents the results of taking the deriva-tive of the best fit of tchezipper model tzo the dat,a. The other was obtained by taking the temperature derivative of a three-point Lagrange interpolation through the data. While there are systematic differences between the two derivative curves, the tendencies of both are the same, as can be seen in Fig. 3, representing a log-log plot of the maximum of the derivative curve against the number of base pairs in the oligomers. This last figure is relevant to the analysis discussed below.
In light of the likely relevance of standard scaling no-tions to DNA melting, we have applied finite size scaling to our data. According to finite size scaling analysis, the specific heat of a d-dimensional system with (linear) size L that undergoes a continuous phase transition will take

FIG. 2: Temperature dependence of the derivative of the UV absorption of poly d(A)-poly d(T) at three different molari-ties. Here, the absorption has been normalized so that the area under each peak is one. The full line is obtained fitting the absorption curves with the zipper model and then taking the derivative ("zipper interpolationn), the dotted line is ob-tained using a three-point interpolation of the experimental data. The parameters used for the zipper fits are given in the table. In all cases, the height of the maximum, and the temperature at which this maximum occurs, increase mono-tonically with the size of the oligomer.

FIG. 3: Log-log plots of the maximum of the derivative of the melting curves against the size of the oligomer at the three molarities. The two sets of points refer to two different interpolations of the data, see caption to Fig.2. The best-fit linear regression fit is shown. Also displayed is a line with unit slope, representing a first order transition in the large N limit.
the form
is cancelled as t Ñ> 0. This implies a
specific heat at [l] J. D. Watson, Molecular biology of
the gene (Ben-jamin/Cummings
Pub. Co., Menlo Park, Calif., 1988), 4th ed. [2] R. M.
Wartell and A. S. Benight, Physics Reports 126, [3] M. Y.
Azbel, Physical Review A (General Physics) 20, 1671 (1979), article. [4] A. Noy, D. V. Vezenov, J. F.
Kayyem, T. J. Meade, and C. M. Lieber, Chemistry
and Biology 4,
519 (1997). [5] D. K.
Lubensky and D. R. Nelson, Physical Review Let-ters 85, 1572
(2000). [6] S.
Cocco and R. Monasson, Physical Review Letters 83, 5178
(1999). [7] K. A.
Marx, J. W. Bizzaro, R. D. Blake, M. Hsien Tsai, [8] T.
F. Turner, J. E. Ahlquist, and M. M. White, Jour-nal of Fish Biology 37, 531 (1990),
12-4 FIELD Section Title:Nonmammalian Biochemistry Dep. Zool. Biomed. Sci.,Ohio
Univ.,Athens,OH,USA. FIELD URL: written [9] C. Kittel, American
Journal of Physics 37, 917 (1969). [10] D. Poland and H. A.
Scheraga, J. Chem. Phys. 45, 1456 (1966).
[11] D.
Poland and H. A. Scheraga, J. Chem. Phys. 45, 1464
(1966). [12] D. Cule
and T. Hwa, Physical Review Letters 79, 2375 (1997), article Aps
Access restricted. [13] N. Singh
and Y.Singh, Physical Review E: Statistical, Nonlinear, and Soft Matter Physics
64, 042901/1 (2001). [14] Y.
Kafri, D. Mukamel, and L. Peliti, Physical Review Letters 85, 4988
(2000). [15] M.
Peyrard and A. R. Bishop, Physical Review Letters [16] In its
simplest form, this family of models takes only par-tial account of the effects
of excluded volume and ignores the influences of base-pair inhomogeneity and
torsional strain associated with the formation of denaturation bub-bles. Much
of the recent theoretical literature in DNA melting is concerned with attempts
to take proper ac- [17] C. R.
Cantor and P. R. Schimmel, Techniques for the [18] E. Kauffman, G. Gmner, and G.
Zocchi (2002). [19] C. R.
Cantor and P. R. Schimmel, The behavior of bao-logical macromolecules (W. H.
Freeman, San Francisco, 1980). [20] C. R.
Cantor and P. R. Schimmel, The conformation of biological
macromolecules (W. H. Freeman, San Fran- ; 24 cm.
![]()
![]()
in the immediate vicinity of the transition. In the
above expression, t is the reduced
temperature and v is the cor-relation
function exponent. As no finite system exhibits thermodynamic singularities,
the behavior of the func-tion f is
such that the singularity in t in the
prefactor
the bulk transition temperature that goes as L(2-dv)/v.
In the case at hand, d = 1, so the specific heat at the
bulk transition temperature scales as L(2-v)/v. This sort
of dependence on L also
characterizes the maximum in the specific heat. The temperature derivative of
the UV absorption should behave in essentially the same way
as does the specific heat at the denaturing transition, against L, and we have evaluated the
maximum values
of dn/dT using two procedures. The first involves the Lagrange interpolation
through the data. The second, which we call the "Zipper
interpolation" refers to a theo-retical fit to the observed temperature
dependence, using Eq. (I),with â‚ as a free parameter for each oligomer and
identifying the maximumof the derivative of the fit. The length dependence of
the maxima are displayed in Fig. 3, and we find that the optimal fit is
consistent with a spe-cific heat that scales as L0.86 which implies a v = 1.075 and a specific
heat diverging as ![]() in an infinite system.
Consequently, the transition is continuous, but close to first order. Our
findings are, therefore, consis-tent with the picture that bubble formation. in
addition to opening at the ends, is important for the denaturing process. This
is so even in the case of relatively short oligomers.
in an infinite system.
Consequently, the transition is continuous, but close to first order. Our
findings are, therefore, consis-tent with the picture that bubble formation. in
addition to opening at the ends, is important for the denaturing process. This
is so even in the case of relatively short oligomers.
The experiments and analysis given above lead to
sev-eral conclusions. First, it is clear from Fig 2 that the zip-per model is a
better fit to the experiments than the two state model for longer oligomers.
This is in agreement with observations [17]that the dependence of the melting
temperature on oligomer concentration is not significant above a length of
about 14, while for shorter oligomers the transition is a chemical equilibrium
between single strand and duplex species, which depends on concentra-tion [20].
Contrasting our results obtained on different lengths suggest that bubble
formation, and a scaling sce-nario of the denaturing transition, is likely to
be impor- tant. Experiments on longer oligomers, where the bub-
ble formation process is expected to be more important would be desirable in
order to distinguish between the zipper model and phase transition scenario. Finally
we note that we have analyzed only the average, or mean number of open base
pairs. The measurements described here do not offer insight into effects
associated with fluc-tuations.![]()
and L. Feng Tao,Molecular and Biochemical Parasitol-
ogy 107, 303 (2000).
in English.
62, 2755 (1989), article.
count of the above and other effects on DNA melting.
study of biological structure and function (W. H. Free-
man, San Francisco, 1980).
cisco, 1980), charles R. Cantor, Paul R. Schimmel. ill.