In Situ Coherent Diffractive Imaging
[Y. H. Lo, L. Zhao, M. Gallagher-Jones, A. Rana, J. J. Lodico, W. Xiao, B.C. Regan and J. Miao, "In situ coherent diffractive imaging", Nat. Commun. 9:1826 (2018).]
Posted on May 8th, 2018
Coherent diffractive imaging (CDI) has been widely applied in the physical and biological sciences using X-ray and optical laser. One of CDIs important applications is to probe dynamic phenomena with high spatiotemporal resolution. In this work we introduced a general in situ CDI method for real-time imaging of dynamic processes in solution. By exploiting a time-invariant overlapping region as real-space constraint, we simultaneously reconstructed a time series of complex exit wave of dynamic processes with robust and fast convergence. We validated this method using optical laser experiments and numerical simulations with coherent X-rays. Our numerical simulations further indicated that in situ CDI can potentially reduce radiation dose by more than an order of magnitude relative to conventional CDI. With further development, we envision in situ CDI could be applied to probe a wide range of dynamic phenomena in the future.
Simulated Pb dendrite growth dynamics with 8 keV X-ray
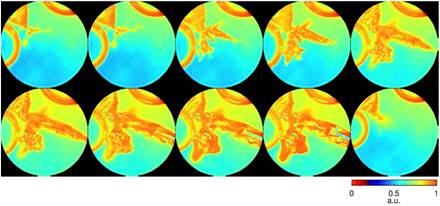
We performed numerical simulations on real time imaging of Pb dendrite growth in solution. Coherent X-rays with 8 keV energy and a flux of 1011 photons μm-2 s-1 were incident on a dual-pinhole aperture. The illumination function was generated by propagating an exit wave from the dual-pinhole aperture to the sample plane. One of the pinholes illuminated the growth process of Pb dendrites immersed in a 1-μm-thick water, while the other pinhole was focused on a static region. A time series of diffraction patterns were collected by a 1024×1024 pixel detector with a frame rate of 100 Hz and a linear oversampling ratio of approximately 2. Poisson noise was added to each diffraction pattern and the central 5×5 pixel data was removed to simulate the missing center problem.
Experimental Pb dendrite growth dynamics with optical laser
Pb dendrite
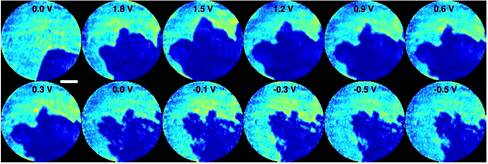
The growth of Pb dendrites on Pt electrodes immersed in an aqueous solution of Pb(NO3)2 was captured using a helium-neon (HeNe) laser. The coherent light source illuminated a dual-pinhole aperture composed of two 100 μm holes spaced 100 μm apart edge-to-edge, and an electrochemical cell was placed 400 μm downstream of the aperture. The cell was made from 50 μm diameter Pt wires immersed in 1.5M Pb(NO3)2 solution and encased between two 100-μm-thick coverslips. In the dataset, the left pinhole was placed in front of the electrochemical cell, while the right pinhole was focused on the substrate devoid of any dendrite. Twelve DC voltages were applied to the electrochemical cell to generate Pb dendrite growth and dissolution. At each voltage, a diffraction pattern was measured by a liquid-nitrogen cooled CCD detector with 1340×1300 pixels and a pixel size of 20×20 μm.
Glioblastoma
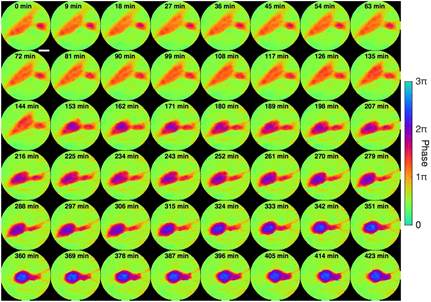
Tumor cell interactions offers insights into cancer progression, including recognition, communication and assembly among cell groups. Tumor cell fusion, or fusogenic events, has also been suggested as a source of genetic instability, as well as mechanisms for metastasis and drug resistance. The dynamics of tumor cell interaction and fusion was captured using the same experimental setup as above. To demonstrate in situ CDI in a biological context, we used a HeNe laser and collected a time series of 48 diffraction patterns from live glioblastoma cells sealed between two cover slips.
Dose reduction
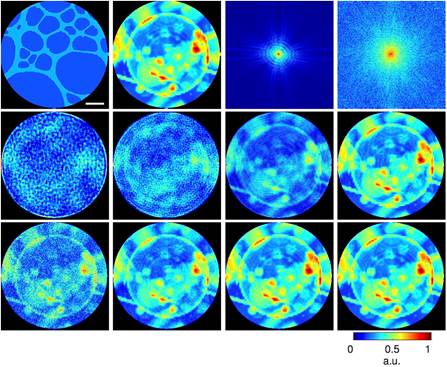
To examine the feasibility of dose reduction using an auxiliary scattering enhancing structure, we simulated a static structure of a 20-nm-thick Au pattern and a biological sample consisting of a vesicle and protein aggregates. Both the static structure and biological sample are submerged in 1-µm-thick H2O and masked by a 3 µm pinhole. The diffraction patterns were collected by a detector with quantum efficiency of 80% and Poisson noise was added to the diffraction intensity. Using coherent soft X-rays (E = 530 eV), we first calculated the diffraction patterns only from the biological sample with a total fluence varying from 3.5×104 to 3.5×107 photons µm-2 (2nd row), corresponding to a radiation dose ranging from 2.75×103 to 2.75×106 Gy, respectively. Then next to the same cellular structure we calculated diffraction intensities with diffusive structure (3rd row).
Software and data
For those who are interested in our method, we make the data, image reconstruction and data analysis source codes in Matlab freely available below.
Download the codes.zip file of reconstruction codes here (12 KB). It contains:
1. Main.m run this main file to reconstruct all datasets
2. isCDI.m in situ CDI reconstruction code
3. batch_oss.m dose reduction batch OSS reconstruction code
4. crop_roi.m crops images for display purposes (auxiliary)
5. FFT_operator class for performing FFT on a stack of images simultaneously. Add folder to Matlab search path
Download the data.zip file of data here (357 MB). It contains:
1. sim_data_pb_dendrite.mat simulated 8 keV X-ray Pb dendrite growth dataset
2. exp_data_glioblastoma.mat optical laser glioblastoma cell dynamics dataset
3. exp_data_pb_dendrite.mat optical laser Pb dendrite growth dataset
4. sim_data_dose_reduce.mat simulated dose reduction of biological structure dataset
This document was prepared by Yuan Hung Lo, Yongsoo Yang and John Miao in the Department of Physics & Astronomy and California NanoSystems Institute, University of California, Los Angeles, California, 90095, USA. Email: miao@physics.ucla.edu.